- HOME
- Calcium carbonate Museum
- CaCO3 in our life field
You can find CaCO3 everywhere in your life stage and used for many things. It can be said CaCO3 is just like good supporting role of our contemporary life. Because limestone(CaCO3) is entirely harmless, white, not expensive and produced huge amount, CaCO3 is used every where. Let’s take a look how exist CaCO3 in nature and how used in our life.
CaCO3 in nature

1.Calcium carbonate was formed by inner energy of Earth.
CaCO3 is changed its shape by the diastrophism, heat, pressure, etc.
As mentioned previous chapter there are calcite, aragonite and low crystallinity marble and limestone as calcium carbonate mineral crystals. Calcite has high refractive index, so sliced calcite give a beautiful light same as jewels, but calcite is soft and its hardness is very low, so that calcite is no practical use as a jewel.
the sourceAs mentioned previous chapter there are calcite, aragonite and low crystallinity marble and limestone as calcium carbonate mineral crystals. Calcite has high refractive index, so sliced calcite give a beautiful light same as jewels, but calcite is soft and its hardness is very low, so that calcite is no practical use as a jewel.
1) Gunma Museum of Natural History (http://www.gmnh.pref.gunma.jp/)
2) Virtual Museum of Gems & Gem Crystals (http://www15.plala.or.jp/gemuseum/)
2.Life-form create calcium carbonate.

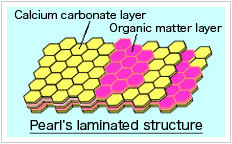
CaCO3 from Mars
CaCO3 for industry
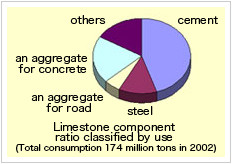
Let’s take a look how CaCO3 is used with concrete examples.
1.Building materials
We the human beings had been using CaCO3 with the progress of civilization. One of the use is building materials. For examples. The pyramid of Egypt was build 5000 years ago. Big limestone its weight is more than 10 tons are well used for building the pyramid. At the present day, there is no need to say limestone and marble are used for building materials. Today, limestone is also a raw material of concrete(cement).
2.Steel
Steel and CaCO3. It seems there are no relations about two materials but both materials have deep relations. Iron ore is a raw material of steel. Iron ore contains only 60% iron, so it needs to remove other impurities from iron ore. CaCO3 react with theses impurities and easy to remove impurities from iron ore. 0.2tons of CaCO3 is used to make one ton of steel.
3.Other
CaCO3 is used in many fields. For example, plastics and rubber products needs CaCO3 to make it stronger and harder and hard to burn. Paper also need CaCO3 to improve surface complexion and prevent to ink sinking into the paper. Further more CaCO3 is employed for soil amelioration as a fertilizer in farming field. And CaCO3 is used for paint, tile, china, food additives, medicine, tooth paste, feed of domestic animals, pigment etc. so CaCO3 is an indispensable material for things familiar close to our life.

The source:
Japan Lime Association
“The story of limestone”p.40(1987)
Japan Lime Association
“The story of limestone”p.40(1987)